Here is a sample that showcases why we are one of the world’s leading academic writing firms. This assignment was created by one of our expert academic writers and demonstrated the highest academic quality. Place your order today to achieve academic greatness.
The drug business of the UK is now globalised to most countries, so the pharma organizations are compelled to re-appropriate and increment their reliance on outsider providers and sellers. Along these lines, the store network continued to fortify in intricacy, and the pharma organizations began to think it is trying to handle the expanding digital dangers.
Furthermore, in light of the episode of covid19, there were many dangers looked by the drug business of the UK, and the pharma organizations anticipate dealing with those dangers and moderate their effects on their fundamental items and administrations (Clarke et al., 2017). Pfizer, UK, is the organization chosen to examine the danger the executives rehearses gained to manage the dangers before and during covid19.
Pfizer is a worldwide drug organization devoted to finding, assembling, and market professionally prescribed prescriptions for people and creatures. As indicated by the organization’s authorities, there were some huge difficulties and dangers that the organization needed to experience. A few dangers were distinguished as the organization was confounded in overseeing hazard and issues, and both were not contained similarly.
The organization emerged from this disarray when social contrasts and the innovative points of view of the issues confronted were clarified to the organization staff (Fan et al., 2018). Along with Pfizer, other pharmaceutical companies operating in the UK have seen to implement effective risk management, which leads to strong outcomes in terms of performance and growth. Furthermore, training and division of responsibilities are considered a practical risk management approach followed by many organizations worldwide.
Similarly, Pfizer, the UK, has faced some significant risk, which was managed through practical risks management practices and investigation. This is the primary aim of this study. Furthermore, this study is critical because, through this systematic review, other pharma companies might find some risk management practices practical to manage their risks. This research study will provide a baseline for future studies by providing the risk management approach set by Pfizer and other pharmaceutical companies.
The chief point of this examination is to research how the drug business of the UK adapts to the risks and difficulties confronted and how they deal with the risks in an unexpected way. This examination will primarily focus on the pharma organization of the UK named Pfizer. Accordingly, the chief point of this exposition is to investigate the functional risks management practices gained by Pfizer to oversee basic risks, including the effects of covid19 and others. One more essential point of this examination is to prescribe the executives practices and strategies to Pfizer in the wake of surveying the issues and risks looked by the organization.
This study will review existing literature systematically, which means that secondary research will be conducted. The primary reason for employing secondary research is because of its cost-effective nature, as well as it is less time-consuming by still delivering the set of practical results based on observations, literature extraction, and focus groups. Therefore, the qualitative secondary research will take place in which the datasets will be identified after being selected from the past studies on the risk management practices acquired by Pfizer and other pharmaceutical companies.
The exploratory research design is selected for this investigation process which is enormously used to assess the research problems for which little or no prior knowledge is needed. Furthermore, this study will be completed in four significant steps. Firstly, the research questions will be developed. In the second stage, the evaluation of those studies will take place and will not include the findings and conclusion of the study.
Instead, this step will only cover the introduction and discussion of the contexts of datasets. The final stage of this study will target the preparation and analysis of the data collected from different sources. In this stage, the research questions will be answered based on identified themes and patterns in multiple selected studies.
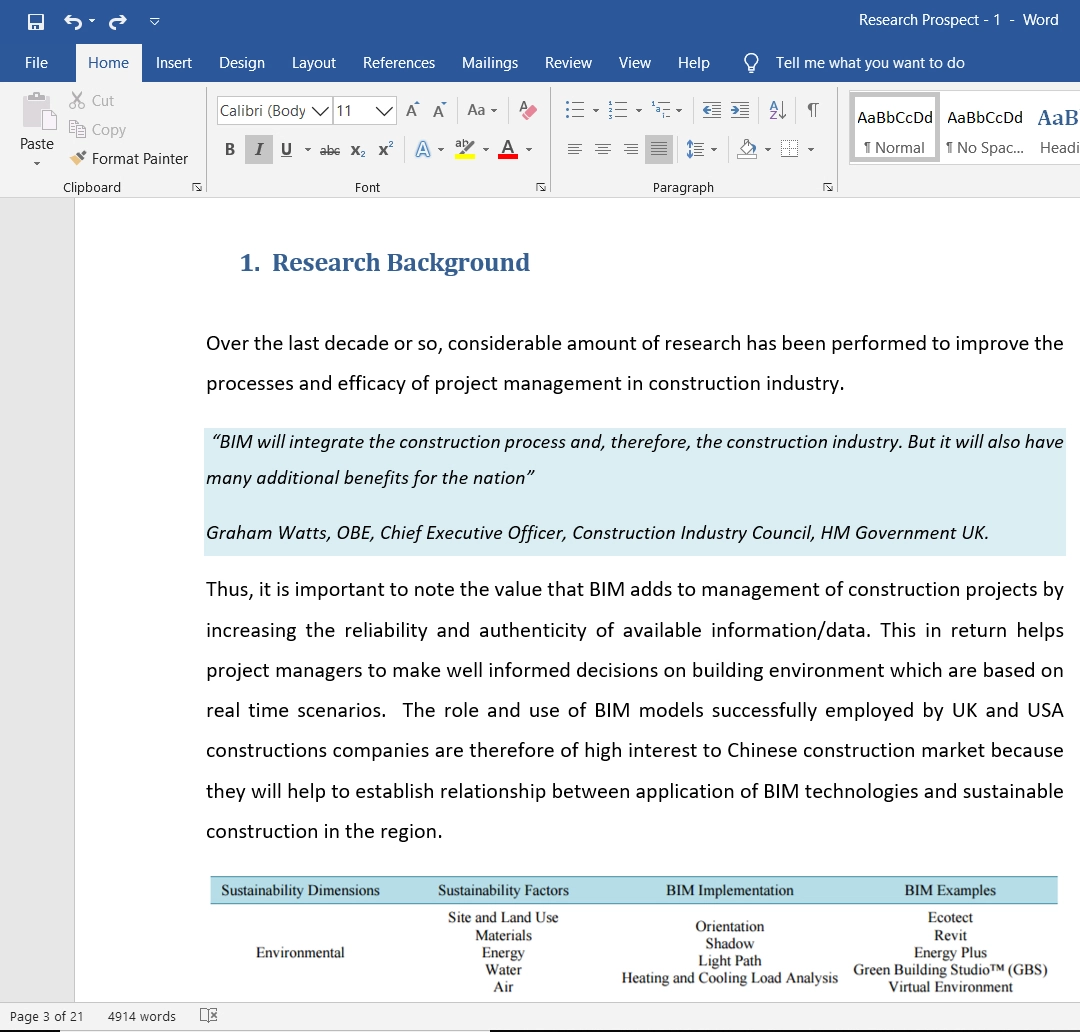
Based on the research background and the objectives of this study, the theoretical framework can be developed. The relationship of risk management strategies to the high performance of the pharmaceutical companies, specifically Pfizer, UK, will be investigated. This relationship can be illustrated as follows:
Figure 1- Theoretical Frame of the Research Study
The second chapter will review multiple research articles and empirical studies targeting the risk management practices acquired by UK-based pharmaceutical companies. Furthermore, chapter three will base on the meticulous assessment of the projected methodology used to determine the study findings. The next chapter will thoroughly appraise the identified study dataset, and the data gathered from those sources will be analyzed critically based on standard assumptions. This chapter will mainly base on the analysis of findings of the selected datasets. The last chapter of this dissertation will conclude the study’s results and will lead to vast recommendations for future studies.
As per the depiction from Giannakis et al., 2016, successful and quality-fostered risk management is referred to as a process for examination, control communication, and the detailed review of the risks in terms of quality of the pharmaceutical products throughout the product’s lifecycle. Furthermore, it is also claimed that the pharmaceutical companies observe numerous risks.
Many companies released reports on the dangers they encounter and the strategies they took to mitigate those risks’ impacts (Bucalo et al., 2017). Some of those risks include rapidly growing competition, pharmaceutical fraud, increasing consumer expectations and challenges while managing the health of a brand, supply chain disruptions, data breaches, and other cybersecurity threats.
Furthermore, it is claimed through a study by Muktadir et al., 2018 that risk management practices have widely been followed within numerous businesses and industries, including pharmaceutical companies, through which risk assessment and evaluation occur. It is evident by a researcher that in pharma companies, risk management is appropriately used in healthcare. It is used to report incidents and frauds and to minimise the harm that clinical or resourcing errors can cause to staff, patients and the pharma stakeholders.
As indicated by Huq et al., 2016, pharma organizations are liable for considering the patients and securing patients by overseeing risks as far as quality frameworks and assembling measures. Each course of medication creation and different items are connected with various dangers, because of which the nature of an item should be kept up with all through the item lifecycle (Jareb et al., 2017).
Edges et al., 2016 have recommended that, inside the monetarily and politically inclement climate, the pharma organisations look at many risks regarding the clinical preliminary plan and execution, item quality, drug endorsement, just as worldwide business rehearses. To deal with this load of risks, useful risk management approaches are required that can prompt different development openings.
Moreover, it is likewise guaranteed that most pharma organizations of the UK have been confronting a difficult danger climate because of which quick fall has been seen in the organization esteem. Around 25% decline has been seen in the conventional medication makers, 30% fall in the biotech organizations of the UK in the previous two years (Silva et al., 2020).
According to the report distributed by Pfizer, UK, it was asserted that the organization has been anticipating executing proficient risks management practices in which they have presented not many prescient components which will relieve the effect of the risk alongside the further advancements of the innovation (Hubbard, 2020).
Moreover, Pfizer and other pharma organizations anticipate zeroing in on things that matter most and viably applying assets. According to Mandhere et al., 2018 it is significant to realize the drugs or medicine’s quality needs to be maintained throughout its lifecycle, such as their attributes that are vital to the quality of drug (medicinal) product remain consistent with those used in the clinical studies.
Furthermore, an emphasized and effective quality risk management plays a significant role in making the pharma business successful by helping the pharma organizations facilitate the consumers at a better place and to make better decisions. Practical risks management provides the manufacturers, regulators, and suppliers with the high-end assurance of the organization’s ability to manage and deal with the potential risks which might occur and can successfully impact the extent and level of the direct regulatory oversight (Chapman, 2019).
As suggested by Fendler (2019), training and development play a significant role among multiple effective practices of risk management followed by Pfizer and other pharmaceutical companies. According to this area of risk and quality management, the company invests in the education and learning of pharma staff and managers.
It is evident through a study that when pharmaceutical companies develop training programs to support their QRM activities. They align their instructions and procedures to be drawn up, which clarifies their strategies and defines the tasks of all personnel taking part in those activities. In this way, training plays a significant role in assuring risk management within pharma companies and other industries.
Furthermore, in a study given by Kumar et al., 2019 some recommendations were given regarding the effective management of risks within the pharmaceutical organization. According to this study, specific training and developmental programs should be initiated which provide and enhance awareness among people regarding identifying and dealing with certain risks.
Also, the staff members and managers should be responsible for managing and reviewing the risks. This is only possible if they receive adequate training in multiple relevant procedures. Among different risk management strategies and approaches, the realization of responsibilities and their equal distribution among people is important.
According to Bhattacharya et al., 2015, successful risk management and application of QRM relies on the clear comprehension of responsibilities by all personnel involved in the QRM activities, which means that every person linked with the development and delivery of pharma products are responsible for managing risks and this is the significant way through hazards are resolved efficiently and timely.
The researcher has recommended that a cross-functional matrix of assigned responsibilities be drawn up and shared with all the entities in an organization. Furthermore, the pharmaceutical manufacturers and suppliers should guarantee that accurate knowledge and expertise are available to effectively plan and accomplish QRM events.
Orders completed by our expert writers are
The research design for any study mainly brings up as a conductor or a blueprint that guides the implementation of a study. Thus, a research design employed for this study is an ‘exploratory research design.’ This research design is widely seen to be consumed by past researchers to discover a research problem requiring little or no previous information or knowledge.
Furthermore, this sort of research design is experienced to be relatively supple, formless, and qualitative as there are several exploratory research methods, including pilot studies, surveys, secondary data, focus groups, in-depth interviews. For the current research study, secondary data analysis is selected as an exploratory research measure.
The chief steps that will be taken further in this research include pattern recognition within selected studies of the identified dataset, observation of multiple themes, and reporting the way of explanations followed by the authors. After all the above-stated steps are accompanied within the exploratory study, conclusions can be drawn, and recommendations can be drawn for future studies.
The significant questions that are going to be answered through this research study are as follows:
Dataset identification is the second phase of this secondary research study after developing comprehensive and realistic research questions. Underneath, a dataset comprising of two studies is mentioned that will be cast off for the current investigation of the best risk management practices acquired by Pfizer and other pharma companies operating in the UK. The selected studies’ methodology, topics, discoveries, and debate might vary. Still, while electing the dataset to be analyzed critically to accomplish study objectives, their main idea and theme were guaranteed to be similar.
The first study of the identified dataset targets secondary investigation of ‘risk management in National Health Services (NHS): governance, finance, and clinical risks” (Fenn et al., 2012). This study has targeted NHS, the publicly funded healthcare system in the UK, which is also connected to different healthcare sectors, including Pfizer.
This study has effectively explored the governance and finance risks faced by NHS and the clinical implication in response to different risks. On the other hand, the second study of the dataset is based on the “Quality Risk Management (QRM) principles and industry case studies” (Frank et al., 2008). This study has followed the case study research design within secondary research to investigate different practices acquired by the pharma companies in order to manage the risk related to the quality and supply of the medicines and drugs.
This part of the study refers to how the required data will be gathered from different sources on the basis of certain criteria. Before analyzing the different aspects and dimensions of selected studies critically, the vitality and individuality of the studies will be assessed first. For this purpose, some imperative questions will be answered in the next section of this dissertation.
The major intention behind countering those questions before evaluating the studies and deducing the results is to authenticate the methodologies acquired, their research origins, and their significance. The questions below will help evaluate findings later on when their answers will be compared for data analysis.
Exploratory research will assist in evaluating the information and conveying expected results. Thus, for this, the acquaintance with the actual research studies must be expanded through the evaluation and data analysis process. Further in the dissertation, the data patterns and themes of discussion will be examined so that a precise set of conclusions and genuine recommendations can be drawn to answer the research questions of this study. The results of all the selected datasets will be analogized to protect the knowledge among aspects and draw reasonable conclusions.
To succeed with this dissertation, one of the significant imperative aspirations is to conclude ethically. Initially, it was guaranteed that no one’s opinions or choices towards the research are going to be hurt. A similar thing was deliberated while categorizing the dataset for the dissertation. In this regard, the authorization of the authors of selected studies was preferred.
The above sections of this dissertation have effectively covered the literature explaining the background and past studies conducted under the current research topic which helped in developing the understanding of the risk management practices within the pharmaceutical sector of the UK and specifically the company named Pfizer, UK.
During the step of dataset identification for this secondary research, it was perceived that past researchers limited their investigation to certain themes like risk management, project management, etc. Most studies did not target a certain company or a specific industry. Therefore, this dissertation is going to target a specific company named Pfizer, UK and the selected research studies will be analyzed to learn how Pfizer and other pharmaceutical organizations are looking forward to implementing effective risk management practices.
From the above chapter of this dissertation, it was evident that in order to endure with the secondary research, some questions will be answered to assess the reliability, validity, and effectiveness of the selected studies. The major rationale of including these questions is to relate the aims and objectives of the current dissertation with the objectives of selected studies for the secondary research. Therefore, after these questions are answered, the research study will move on towards discussion of the selected studies through which different study patterns and themes will be identified and assembled to reach the conclusion of the study.
This systematic research paper aimed to explore a certain theme of risk management by investigating the inter-relationship of financial incentives, better governance, and clinical risk management practice followed within the healthcare sector of the UK including Pfizer, UK. Another aim of this study was to provide an overview of the recent developments together by gathering evidence through literature search expressed by the decision-makers within the NHS. This study aimed to review the quality of risk management practices acquired by the NHS and how Pfizer, other pharma companies, and hospitals.
A secondary approach of data collection was used in this paper which is referred to as, ‘literature search strategy’.
The PQRI-MTC sponsored the risk management working groups to accumulate industry case studies with the aim of advancing the comprehension and application of ICH belonging from different risk management expertise and experiences. The principal aim of this study is to explore the outcomes of the risk management working group and provide a summary of mutual risk management best practices and principles along with different working tools to foster consistency around the usage of day-to-day risk management and decision-making practices. This research study also objectified to investigate the list of examples of risk management applications used in different pharmaceutical firms of the UK including Pfizer.
This study has employed the ‘case study research design’ that is referred to as the in-depth investigation of certain research problems in place of statistical surveys/interviews or comprehensive comparative inquiry.
After accomplishing the major milestone of accumulating data which proved the effectiveness and accuracy of the identified studies of the dataset, this section of the data analysis is going to include a final evaluation of the study findings on the basis of definite themes and patterns through the argument of results gathered through them.
Later on, different research patterns and the study discoveries will be matched and in the last stage of the study, the research questions will be responded to ultimately. It is observed that secondary information from all the selected studies of the dataset was of high quality which means that data was gathered from efficient sources and is not outdated. Furthermore, the measures employed for the studies in order to conclude their results were effective that provide the reliability of the selected studies.
Effective risk management according to this research study is referred to as the wide area of clinical ascendency followed by Pfizer under NHS, through which NHS organization become answerable for the continuous enhancement of service’s quality and fortification of high standards of the case by developing an environment in which distinction in the clinical care can thrive. Another theme of this study leads to diverse facets of risk management in terms of clinical governance including risk guidelines, risk reporting to auditing teams, risk assessments, and training.
Pfizer, UK has been observed to adapt to changing environments rapidly which leads to some major risks. But the company performed efficiently by helping its stakeholders understand the difference among risks and issues through training and developmental programs as well as by teaching them to depict a positive attitude towards risks. Due to this reason, NHS now proudly claims that more contributors to the clinical trials with skills and understanding of risk management come up to make outcomes positive.
Furthermore, this study has critically discussed the interrelationship of finance, governance, and risk management. The best practices of risk management are related to the effective regulation of governance and finance within Pfizer and other pharma companies. It is claimed that the pharma companies with strong governance and corporate culture perform quite well in terms of managing clinical risks as the resources are allocated accordingly through effective decision making.
At the same time, the pharma companies like Pfizer, etc. with rigorous finances are also anticipated to manage risks effectively. Therefore, this paper has efficiently proved through the strategy of literature search about how and why the practices of strong governance and high-end finance are important for effective risk management within pharmaceutical organizations like Pfizer and NHS. Furthermore, this paper has also emphasized the vitality of communication and internal incentives within the pharma companies that lead to a strong project base without the expectations of uncontrollable risks.
This research article has led to different dimensions of risk management through case study analysis. According to it, quality risk management is a major aspect of pharmaceutical businesses that plays a major role towards acceptable and standard quality system practice to expedite efficient decision-making concerning resource prioritization, risk identification, and risk elimination.
Furthermore, after exploring different case studies of the pharmaceutical companies operating in the UK including different types of risk management approaches, the results proved that risk assessment is needed to be used to evaluate the process of assuring compliance and the resulting prioritization for action and not certainly for a decision making process in order to fulfill applicable regulations of other legal requirements. It has also resulted from the research that, the principal approach of the effective management of risks within the pharma industry is when they are identified, assessed, evaluated, and considered for further mitigation and communication with other team members of a certain project.
Through a case study research design, this study has extensively explored different tools and techniques of risk management followed within pharmaceutical companies of the UK. It was assessed that most of the quality risk management practices among pharma companies including Pfizer are based on scientific and process-specific knowledge which is also related predominantly to the protection and cure of the patient.
Also, best practices of risk management are assured in such companies when they depend on the understanding of underlying science, effective regulations, and linked processes involved with the certain risk under analysis. These practices impose a major impact on the ultimate health of the patients who use the services, medicines, or drugs of that particular company.
Figure 2- Quality Risk Evaluation Pyramid followed in the pharma companies of the UK.
It was evident from one case study that most of the pharmaceutical organizations working in the UK realized the importance of understanding their business curriculum. It is claimed through this study that Pfizer always looks forward to studying its business structure as the company realizes that it will provide the company with potential risks and complete ownership of the results of risk management assessment.
After critically analyzing the selected studies of the dataset, it can be claimed that different approaches are uncovered that are used by Pfizer and other pharmaceutical companies of the UK in order to mitigate the impacts of risks. Before managing the risks effectively, Pfizer is seen to implement effective practices to identify upcoming risks and challenges.
First of all, from the above analysis, it is proved that knowledge of internal business structures and effective communication among stakeholders of the companies are principal strategies to identify underlying controllable or uncontrollable risks. Also, risk assessment is the major step that is even more important than risk management and Pfizer has always put its efforts into documenting the risk management process in order to condense appropriate risk assessment. This always makes the process of risk management more effective and practical. A study analyzed above has depicted the list of risk assessment tools that are widely being used by different industries to identify and manage risks as shown in the figure below.
Figure 3- Common risk management tools
Figure 4 – Advanced risk management tools
The above analysis of the identified studies of the dataset has extensively covered the risks assessment and management approaches employed by Pfizer and other pharmaceutical companies. It is assessed through the assessment that Pfizer is open to understanding its business structure along with the behavior of people in case of risk identification.
Furthermore, the above literature review and the dataset analysis it can be claimed that most pharmaceutical companies including Pfizer are aware of the importance of technology and they hope to continue to improve in terms of technological advancements. Some major approaches are also covered while analyzing the datasets but the practices acquired by Pfizer after covid19 are needed to be assessed.
It is evident that the entire quality management system of the company has been affected but the positive factor is that old risks management practices are still applicable and if they are implemented effectively, the results can be informed of the success and increased performance of this pharmaceutical company. Therefore it is believed that Pfizer and other pharmaceutical companies working in any part of the world are just required to apply the known risk management practices after identifying the risks they encounter and evaluating them concerning their impacts on product protection, reliability of trial tests, and then implementing the appropriate ones.
From the above study and entire evaluation of the past studies and literature as a secondary investigation, it is claimed that the best practices of risk management are related to the effective regulation of governance and finance within Pfizer and other pharma companies. It is claimed that the pharma companies with strong governance and corporate culture perform quite well in terms of managing clinical risks as the resources are allocated accordingly through effective decision making.
From the detailed analysis of selected past studies, it is explored that most of the quality risk management practices among pharma companies including Pfizer are based on scientific and process-specific knowledge which is also related predominantly to the protection and cure of the patient. Also, best practices of risk management are assured in such companies when they depend on the understanding of underlying science, effective regulations, and linked processes involved with the certain risk under analysis.
Clarke, J.E. and Liesch, P.W., 2017. Wait-and-see strategy: Risk management in the internationalization process model. Journal of International Business Studies, 48(8), pp.923-940.
Etges, T., Karolia, K., Grint, T., Taylor, A., Lauder, H., Daka, B. and Wright, S., 2016. An observational postmarketing safety registry of patients in the UK, Germany, and Switzerland who have been prescribed Sativex®(THC: CBD, nabiximols) oromucosal spray. Therapeutics and Clinical Risk Management, 12, p.1667.
Fan, Y. and Stevenson, M., 2018. A review of supply chain risk management: definition, theory, and research agenda. International Journal of Physical Distribution & Logistics Management.
Hubbard, D.W., 2020. The failure of risk management: Why it’s broken and how to fix it. John Wiley & Sons.
Huq, F., Pawar, K.S. and Rogers, H., 2016. Supply chain configuration conundrum: how does the pharmaceutical industry mitigate disturbance factors?. Production Planning & Control, 27(14), pp.1206-1220
Jereb, B.J. and Bucalo, N.B., 2017. Risk Management in the Pharmaceutical Industry in Slovenian Companies.
Silva, J., Araujo, C. and Marques, L., 2020. Siloed Perceptions in Pharmaceutical Supply Chain Risk Management: A Brazilian Perspective. Latin American Business Review, 21(3), pp.223-254.
Giannakis, M. and Papadopoulos, T., 2016. Supply chain sustainability: A risk management approach. International Journal of Production Economics, 171, pp.455-470.
Bucalo, N. and Jereb, B., 2017. Risk management in the pharmaceutical industry in slovenian companies. Logistics & sustainable transport, 8(1), pp.42-49.
Moktadir, M.A., Ali, S.M., Mangla, S.K., Sharmy, T.A., Luthra, S., Mishra, N. and Garza-Reyes, J.A., 2018. Decision modeling of risks in pharmaceutical supply chains. Industrial Management & Data Systems.ns.
Mandhare, T.A., Khuspe, P.R., Nangare, P.S. and Vyavhare, R.D., 2018. Quality Risk Management: A Review. American Journal of PharmTech Research, 8(2), pp.56-86.
Fendler, W.P., Calais, J., Eiber, M., Flavell, R.R., Mishoe, A., Feng, F.Y., Nguyen, H.G., Reiter, R.E., Rettig, M.B., Okamoto, S. and Emmett, L., 2019. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA oncology, 5(6), pp.856-863.
Kumar, A., Zavadskas, E.K., Mangla, S.K., Agrawal, V., Sharma, K. and Gupta, D., 2019. When risks need attention: adoption of green supply chain initiatives in the pharmaceutical industry. International Journal of Production Research, 57(11), pp.3554-3576.
Bhattacharya, J., Pharm, M. and Phil, M., 2015. Quality Risk Management–Understanding and control the risk in pharmaceutical manufacturing industry. International Journal of Pharmaceutical Science Invention, 4(1), pp.29-41.
Fenn, P. and Egan, T., 2012. Risk management in the NHS: governance, finance and clinical risk. Clinical medicine, 12(1), p.25.
Frank, T., Brooks, S., Creekmore, R., Hasselbalch, B., Murray, K., Obeng, K., Reich, S. and Sanchez, E., 2008. Quality risk management principles and industry case studies. Pharm Qual Res Inst Manuf Technol Comm [Internet], pp.1-9.
The time to write an undergraduate full dissertation varies, but it typically takes several months, including research, drafting, and revisions.